Solidification is used to transform the waste into a durable physical form that is more suitable for storage, landfill, or reuse. Solidification processes sometimes employ chemical reactions, but are more often absorption. It creates barriers between waste components and the environment by either reducing permeability of the waste, reducing the effective surface area available for diffusion, or both.
Stabilization refers to a chemical technique used to render a waste less toxic or less harmful to the surrounding environment. It reduces the hazard potential of the waste itself. Examples of stabilization techniques include the ion exchange of heavy metals in the alumino-silicate matrix of a cementitious stabilization agent and the sorption of heavy metals on fly ash in aqueous systems. Nonchemical solidification includes dewatering, mixing with adsorbents, and vegetative stabilization. Because these techniques involve adding material to waste (and thereby goes contrary to a general principles of waste management), they increase the weight and volume of waste. Sometimes the increased inertness and stability can justify this mass and volume increase.
Encapsulation is particularly useful for disposal or sharps, or any object that might become a sharp. It is also good for disposal of pharmaceutical residues and some hazardous wastes. A big advantage of the technique is its effectiveness in reducing the risk of scavengers gaining access to the hazardous medical waste.
There are many solidification and stabilization techniques used for different types of waste materials. Generally, stabilization/solidification techniques can be divided into the following six major categories:
 Portland cement is one of the most widely used building materials and is employed throughout the world. Most hazardous wastes can be incorporated into a waste-cement system. The suspended pollutants are incorporated into the final hardened concrete. During this solidification process, the concrete formation binds and strengthens the mass, coats and incorporates some contaminant molecules in the siliceous solids, and block pathways between pores. Due to the elevated pH of cement mixtures (in the range of 9–11), most multivalent cations are converted into insoluble hydroxides or carbonates. Thus, this process is highly effective for waste components with high levels of toxic metals.
Portland cement is one of the most widely used building materials and is employed throughout the world. Most hazardous wastes can be incorporated into a waste-cement system. The suspended pollutants are incorporated into the final hardened concrete. During this solidification process, the concrete formation binds and strengthens the mass, coats and incorporates some contaminant molecules in the siliceous solids, and block pathways between pores. Due to the elevated pH of cement mixtures (in the range of 9–11), most multivalent cations are converted into insoluble hydroxides or carbonates. Thus, this process is highly effective for waste components with high levels of toxic metals.
These techniques involve mixing a siliceous (pozzolanic) material with other alkaline earth material such as lime or gypsum in the presence of water to produce a concrete-like mass. The most common pozzolanic materials used in the stabilization/solidification of wastes are fly ash, ground blast-furnace slag, and cement-kiln dust. Combined cement-pozzolanic processes can be used to give a sturdier and cheaper final waste product.
The process calls for cubic boxes made of high-density polyethylene or metallic drums to be filled three-quarters with waste. The containers are then filled up with a medium such as plastic foam, bituminous sand, lime, cement mortar, or clay. A common recipe is 10% lime, 10% cement, and 5% water.
One piece of equipment used in many encapsulation and immobilization processes in the Dual Planetary Orbital Mixer. Some come ready to be used inside a 55-gallon drum. The motor sits above the drum and the two mixing shafts extend into the waste. The shape of the helical blades/spindles ensures a good mix, even if the material is highly viscous. The shafts are withdrawn before hardening occurs.
When using a thermoplastic process, the waste is dried, heated, and then dispersed through a heated plastic system. Bitumen is the most common matrix material. However, paraffin and polyethylene have also been employed.
Sorbent techniques involve the use of clay minerals or modified clay minerals with high specific surface areas to fix and immobilize hydrocarbon molecules. This immobilization usually takes place on the outer and inner surface of clay particles. Typical clay minerals used in this technique include sodium-montmorillonite and vermiculite.
Organic polymer techniques were introduced to solidify/stabilize radiological wastes to allow for transportation. The urea-formaldehyde (UF) system is the most widely employed organic polymer solidification technique. In this technique, the wet or dry waste is mixed with a prepolymer in a waste secondary containment system. After the two components have been thoroughly mixed, a catalyst is introduced. This process forms a mass that traps the waste. After curing, the polymerized mass becomes highly impermeable.
Encapsulation means coating the waste with inert materials. The coating materials are chemically stable, adhere to the waste, and resist biodegradation. High-density polyethylene (HDPE) and polybutadiene are most often used to perform encapsulation. Designers pay attention to the ratio of waste to insert materials; if that ratio goes to high there is increased risk of waste escaping.
Engineers have tried using a Chemically Bonded Phosphate Ceramic Process - in which the waste is mixed with water, monopotassium phosphate, magnesium oxide and inert additives.
A Sulfur Polymer Stabilization/Solidification has been used for tough-to-treat mercury containing waste.
Inertization is a generic term, and most encapsulation is also intertization. Inertization is often used for ash (from incineration of wastes) and pharmaceutical waste.
One great thing about inertization is the low cost of the equipment and relative ease of use. Workers should wear PPE, but other than that, not a lot is required. Concrete mixers are ubiquitous - finding a used electric one is pretty easy. A crushing machine may be employed to grind the raw waste to smaller particle sizes. Bins or feeders may be employed to hold the lime and cement.
The waste cement can go into almost any sturdy container. That’s one of the beauties of cement - it’s like liquid rock until it hardens. The disposal facility might have rules on what kind of containers you can use. But within reason (don’t make the containers too big), they will probably take anything.
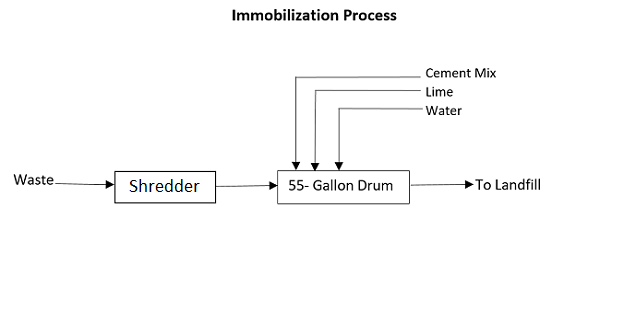
55-gallon drums are often used. They can be metal or plastic (high-density polyethylene). They are cheap and easy to get and interchangeable. You don’t get locked into a drum provider. Drum rings and gaskets are interchangeable.
Containers are usually watertight, but not doubled-walled. The cement itself is sufficient to isolate the waste from the environment.
 You do not want large chunks of waste going into the mixer. The more homogeneous the better. Small pieces of solid waste have a higher surface area to volume ratio than large pieces, and the inertization will be more effective on small pieces. Many process designers install size reduction machines upstream of the cement mixer for this purpose. A “crusher” (types include ball, cone, and gyratory https://www.911metallurgist.com/blog/gyratory-crusher) reduces particle size for pieces that are over an inch in dimension. Rod mills and ball mills can produce even smaller pieces, although at some point the process designer must decide when enough is enough.
You do not want large chunks of waste going into the mixer. The more homogeneous the better. Small pieces of solid waste have a higher surface area to volume ratio than large pieces, and the inertization will be more effective on small pieces. Many process designers install size reduction machines upstream of the cement mixer for this purpose. A “crusher” (types include ball, cone, and gyratory https://www.911metallurgist.com/blog/gyratory-crusher) reduces particle size for pieces that are over an inch in dimension. Rod mills and ball mills can produce even smaller pieces, although at some point the process designer must decide when enough is enough.
Vitrification incorporates the hazardous contaminated waste into a chemical, durable glass which will be stable and acceptable for permanent disposal. When a liquid cools to a solid, the molecules can line up in pattern, which forms a crystal, or settle to an unorganized amorphous mass, which is a glass. The rate of cooling can have a big influence on the final form: rapid cooling doesn’t allow crystals to form and more often results in a glass. Why do we care? Glasses are more stable in the long run and do not permit migration of pollutants through them the way crystals do. The risk of waste constituents leaking at the final disposal site is lower.
Vitrification happens in nature when volcanic magma solidifies to obsidian (silicate glass).. Because of the rapid cooling rate and high liquid viscosity of oxide and silicate, molecules cannot move sufficiently to form a crystalline structure. Hence a glass-like structure is formed. Waste glass is so stable that some vitrified waste, such as municipal incinerator ash, can be made into building materials or insulation. Almost any solid waste can be vitrified into a durable glass. It is most used for radioactive waste, but non-radioactive wastes can also be vitrified.
Vitrification is appealing because it solves many problems. It is expensive, though, and economics dictate it be done on a relatively large scale.
More about vitrification of waste: https://mo-sci.com/vitrification-nuclear-waste-management/
https://www.hanfordvitplant.com/vitrification-101
Containment is used when there is no need to remove the waste material and/or the cost of removal is prohibitive. The main purpose of containment is to prevent or control liquid or semi-liquid contaminated wastes from leaking or leaching into surrounding areas. Techniques include pumping, capping, draining, and the installation of slurry walls. The selection of various types of containment systems is based on the type of waste materials, and geo hydrological conditions of the waste site.
